Getting the pH right is a must in skincare and cosmetics. It's not just a random number—it plays a big part in how well a product holds up, how effective it is, and most of all, how it affects your skin's health.
How Your Skin’s pH Affects Its Function
Your skin naturally has a slightly acidic protective layer on its surface. It's made of sebum, sweat, and other substances from your skin cells, and it keeps your skin's pH in that mildly acidic sweet spot—usually between 4.5 and 5.5.[1]
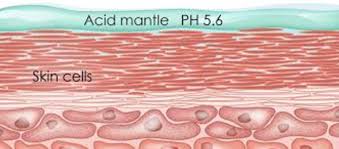
This slightly acidic environment is key to keeping your skin healthy. For starters, it helps your skin's important barrier enzymes work their best. A lot of the enzymes that clear away dead skin cells and create healthy skin lipids do their job best around pH 5.5. If your skin's pH goes up and becomes more alkaline, those enzymes slow down, which can weaken your skin barrier. That leads to moisture loss and problems like dryness, flakiness, and itching.
Your skin can only handle so much when it comes to balancing out pH changes. If you use a product with a very different pH than your skin, your skin has to work overtime to even things out. That extra work can cause irritation. Over time, using products with the wrong pH can wear out your skin's natural balancing act, leaving it more sensitive and likely to get red or develop something like contact dermatitis.
So keeping your skin in its natural acidic zone is really important—it's what sets you up for a strong, hydrated, and resilient skin barrier.
Understand pH: PH Scale: Acids, Bases, and Common Materials
What Influences the pH of a Cosmetic Product?
A product's pH isn't just picked out of thin air. Formulators put real thought into balancing it—making sure the active ingredients actually work, the product stays fresh, it feels nice on your skin, and above all, that it's safe to use. Here's what goes into that decision:
1. The Needs of Active Ingredients
This is probably the most important thing to get right. A lot of the key ingredients in your products only do their job—and stay stable—when the pH is in a very specific range. Take strong, pure Vitamin C (that's L-ascorbic acid), for example. It needs a really acidic setup (below pH 3.5) to stay potent and actually sink into your skin. The same goes for AHAs like glycolic and lactic acid—they need that acidic environment (around pH 3 to 4) to work properly as exfoliants. If the pH isn't right for them, they basically just sit there inactive.
2. The Stability of the Formula
The way a product feels and how well it holds up over time really comes down to its pH. Take a standard thickener like carbomer—it has to be balanced in a slightly alkaline setting to go from runny to gel-like. With cleansers, traditional soap formulas are naturally on the alkaline side (around pH 9-10), but gentler amino acid-based cleansers usually sit in the slightly acidic to neutral range (pH 5.5-7) for a milder touch.
3. The Effectiveness of the Preservative System
Think of preservatives as a product's security team. How well they fight off bacteria and mold really depends on the pH. Most preservatives that come from organic acids—like benzoic acid or sorbic acid—do their best work when things are a little on the acidic side. But once the pH climbs above 6, they start losing their punch pretty fast.
4. The Target Skin Type or User
If a product is made for sensitive skin, babies, or anyone with a compromised skin barrier, its pH needs to be dialed in carefully—right around your skin's natural level (about 5 to 6). Keeping it in that range cuts down on the chance of irritation and actually helps your skin heal itself.
pH Requirements for Common Cosmetic Ingredients
Since pH plays such a big role in cosmetics, we’ve put together a quick guide to the ideal pH range for ingredients you see every day.
1. Acidic Environment (pH ≤ 6.5)
|
Ingredient |
Preferred pH |
|
L-Ascorbic Acid |
2.5 – 3.5 |
|
5.0 – 6.5 |
|
|
Glycolic Acid |
3.0 – 4.0 |
|
Lactic Acid |
3.0 – 4.0 |
|
Salicylic Acid (BHA) |
3.0 – 4.0 |
|
Mandelic Acid |
3.0 – 4.0 |
|
3.0 – 4.0 |
|
|
Protease |
5.0 – 7.0 |
|
Lipase |
6.5 – 7.5 |
|
Kojic Acid |
2.5 – 3.5 |
|
Arbutin |
4.0 – 6.5 |
|
Benzoic Acid & Salts |
≤ 4.5 |
|
Sorbic Acid & Salts |
≤ 6.0 |
2. Neutral to Slightly Acidic Environment (pH 4.5 – 6.5)
|
Ingredient |
Preferred pH |
|
5.0 – 7.5 |
|
|
Glycerin |
4.0 – 8.0 |
|
Urea |
5.0 – 7.0 |
|
Panthenol (Provitamin B5) |
5.0 – 7.0 |
|
Ceramides |
5.0 – 6.0 |
|
Niacinamide |
5.0 – 6.0 |
|
Tocopherol (Vitamin E) |
5.0 – 7.0 |
|
Pyridoxine (Vitamin B6) |
5.0 – 6.5 |
|
Acetyl Hexapeptide-8 |
5.0 – 6.5 |
|
Palmitoyl Tripeptide-5 |
5.0 – 6.5 |
|
Copper Peptides |
5.0 – 6.0 |
|
Centella Asiatica Extract |
5.0 – 6.5 |
|
Green Tea Extract |
5.0 – 6.5 |
|
Licorice Root Extract |
5.0 – 6.5 |
|
Witch Hazel Extract |
4.5 – 6.0 |
|
Bisabolol |
5.0 – 7.0 |
|
Dipotassium Glycyrrhizate |
5.0 – 7.0 |
|
Allantoin |
5.0 – 7.0 |
3. Neutral to Slightly Alkaline Environment (pH 6.5 – 8.0)
|
Ingredient |
Preferred pH |
|
Ascorbyl Glucoside (AA2G) |
6.0 – 7.5 |
|
Magnesium Ascorbyl Phosphate (MAP) |
6.0 – 7.5 |
|
Ascorbyl Palmitate |
6.5 – 7.5 |
|
Soap (Fatty Acid Salts) |
9.0 – 10.5 |
|
Amino Acid Surfactants |
5.5 – 7.0 |
|
SLS / SLES |
6.0 – 7.5 |
|
Avobenzone |
6.0 – 7.5 |
|
Octocrylene |
6.0 – 8.0 |
|
Homosalate |
6.0 – 7.5 |
|
Carbomer |
6.0 – 8.0 |
|
Xanthan Gum |
6.0 – 8.0 |
|
Cellulose Gum |
6.0 – 8.0 |
|
Enzymes (e.g., Papain) |
6.5 – 7.5 |
|
Trehalose |
6.0 – 8.0 |
|
Disodium EDTA |
6.0 – 8.0 |
4. Special Notes on Specific Ingredients
|
Ingredient |
Preferred pH |
|
Benzoyl Peroxide |
5.0 – 7.0 |
|
Sulfur |
5.0 – 7.0 |
|
Azelaic Acid |
5.0 – 6.0 |
|
Ammonium Glycolate |
6.0 – 7.0 |
Stanford Chemicals Company (SCC) is a professional supplier of cosmetic ingredients. You can inquire with us for detailed information about these products. Send us an inquiry.