What is the Solubility of Hyaluronic Acid
The solubility of hyaluronic acid (HA) refers to its ability to dissolve in a specific amount of solvent (typically water) under certain conditions such as temperature and pH, forming a uniform solution. Solubility is usually expressed as the amount of HA (in grams) that can dissolve in 100 milliliters of solvent. Solubility is an important characteristic because it dictates the usability and effectiveness of HA in different applications. For instance, in cosmetics and pharmaceuticals, the solubility of hyaluronic acid affects how it interacts with other ingredients, its distribution within the final product, and the overall sensory experience and effectiveness when used.

Fig 1. Solubility of Hyaluronic Acid
Read more: Top 10 Benefits of Hyaluronic Acid
--Factors Influencing the Solubility of Hyaluronic Acid
- Molecular Weight: The higher the molecular weight of HA, the lower its solubility.
- Temperature: Solubility generally increases with higher temperatures.
- pH: Hyaluronic acid is more soluble in neutral or slightly acidic conditions (pH 5-7). Extremes in pH can negatively impact its solubility.
- Type of Solvent: HA has higher solubility in water and saline solutions, whereas its solubility in organic solvents is typically lower.
- Stirring and Time: The rate and duration of stirring during the dissolution process also affect the solubility of HA. Continuous stirring can speed up the dissolution process.
--Optimal Conditions for Dissolving Hyaluronic Acid in Different Solvents
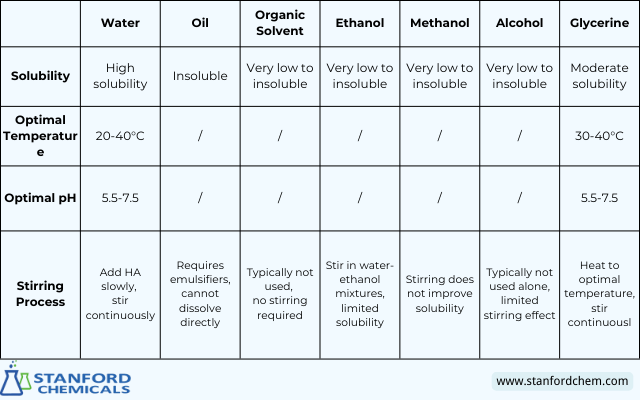
Hyaluronic acid is highly soluble in water and moderately soluble in glycerine, with optimal dissolution occurring at temperatures between 20-40°C for water and 30-40°C for glycerin. The pH range of 5.5-7.5 is ideal for dissolution in both water and glycerine. In contrast, HA is insoluble or has very low solubility in oil, organic solvents, ethanol, methanol, and alcohol, making it less suitable for its use without additional formulation techniques, such as emulsification in oil. The stirring process is crucial for ensuring complete dissolution in water and glycerine, while in other solvents, stirring has limited to no effect. Table 1. outlines the solubility and optimal conditions for dissolving hyaluronic acid in various solvents, including water, oil, organic solvents, ethanol, methanol, alcohol, and glycerine.
Table 1. Optimal Conditions for Dissolving Hyaluronic Acid in Different Solvents
Stanford Chemical Company (SCC) is a trusted supplier of sodium hyaluronate powder. We provide customers with high-purity, non-animal-derived, non-GMO hyaluronic acid powder (including food grade, cosmetic grade, injection grade, medical grade, eye drop grade, cross-linked gel). Enjoy bulk purchase discounts and contract pricing.
How Molecular Weight Affects the Solubility of Hyaluronic Acid in Different Solvents
The molecular weight of hyaluronic acid significantly impacts its solubility in various solvents. Below are some key factors that explain how molecular weight influences solubility.
--Water Solubility
Lower molecular weight means shorter molecular chains and fewer molecular entanglements, which reduces the intermolecular forces that need to be broken during dissolution. As a result, low molecular weight hyaluronic acid generally has better water solubility and dissolves more quickly and completely in water or saline solutions. In contrast, high molecular weight HA has lower solubility in water. It dissolves more slowly, often requiring longer stirring times or increased temperature to facilitate dissolution.
--Solubility in Organic Solvents
Hyaluronic acid generally has poor solubility in organic solvents such as ethanol and isopropanol. Low molecular weight HA, while still not highly soluble in these solvents, is easier to mix with water, allowing it to be used in organic solvent-based formulations. On the other side, high molecular weight HA is even less soluble in pure organic solvents and typically needs to be dissolved in water first before being mixed into an organic solvent.
--Viscosity
High molecular weight HA produces solutions with higher viscosity, which further complicates the dissolution process. Higher viscosity restricts molecular movement within the solution, slowing down the dissolution rate.
--Dissolution Time
Low molecular weight HA dissolves in a shorter amount of time, while high molecular weight requires extended stirring or even ultrasonic treatment to accelerate dissolution.
--Dispersion
Low molecular weight hyaluronic acid disperses more evenly in the solvent, whereas high molecular weight HA may form clumps, increasing the difficulty of dissolution.
--Conclusion
The larger the molecular weight of hyaluronic acid, the poorer its solubility in water and other solvents. This is because of the longer molecular chains of high molecular weight HA, which are prone to forming complex entanglements, making dissolution more challenging. To enhance the solubility of high molecular weight hyaluronic acid, increased stirring time, higher temperature, or auxiliary methods like ultrasonic treatment are often required. 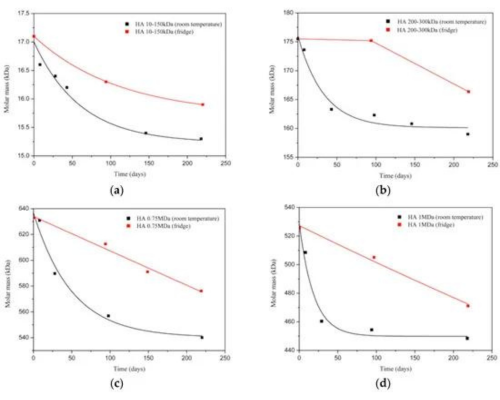
Fig 2. Degradation of HA samples with different MW
Read more: High vs. Low Hyaluronic Acid: How Molecular Weight Affects the Efficacy
The Impact of Hyaluronic Acid Solubility on Its Applications
The solubility of hyaluronic acid is critically important in cosmetic and pharmaceutical applications, directly influencing the product's performance, stability, and effectiveness.
--Impact of Hyaluronic Acid Solubility on Cosmetics
Solubility affects the moisturizing effect of HA. The high solubility of HA allows it to absorb significant amounts of water, forming a moisture-retaining layer on the skin’s surface. This layer not only prevents water loss but also keeps the skin soft and smooth. Therefore, hyaluronic acid is often used in moisturizers, serums, and masks to enhance the product's moisturizing properties.
Solubility affects the texture of HA products. Good solubility enables hyaluronic acid to create uniform solutions or gels in cosmetic products, improving the overall user experience. For example, in lotions or serums, HA can blend well with other ingredients, resulting in a refreshing and lightweight texture that doesn’t feel sticky on the skin.
Solubility affects the delivery of active ingredients. The excellent solubility and permeability of HA make it an effective carrier for active ingredients. In cosmetics, hyaluronic acid can help deliver other active components deeper into the skin, enhancing the efficacy of the product. 
Fig 3. Sodium hyaluronate for skin
--Impact of Hyaluronic Acid Solubility in Pharmaceuticals
In pharmaceutical applications, the solubility of hyaluronic acid is crucial for drug delivery systems. High solubility allows HA to bind with drug molecules, forming stable solutions or gels that help the drug diffuse and absorb better in the body, thus increasing bioavailability.
The solubility of HA makes it suitable for injectable forms of medication, such as joint injections or subcutaneous injections. Well-dissolved hyaluronic acid can be distributed uniformly in the body, enhancing therapeutic effects and reducing adverse reactions.
In controlled-release drugs, the solubility of hyaluronic acid affects the rate at which the drug is released. HA can modulate its solubility to control the drug's release, allowing it to be gradually released in the body, prolonging its therapeutic effect, and reducing the frequency of dosing.
In summary, the solubility of hyaluronic acid plays a critical role in both cosmetic and pharmaceutical applications, influencing product efficacy, texture, user experience, as well as drug delivery and release.
How to Enhance the Stability of Hyaluronic Acid in Solution
The stability of HA in solution is crucial for the performance, efficacy, and user experience of related products. Enhancing its stability is key to the practical application of HA products. Here are some common methods to improve the stability.
- Control pH Levels: Hyaluronic acid is most stable at a near-neutral pH (5-7). Buffer solutions can also be used to maintain the pH of the solution, preventing fluctuations caused by external factors.
- Lower Temperature: Hyaluronic acid is more stable at lower temperatures. Therefore, during preparation and storage, it is advisable to avoid high-temperature processes.
- Use of Antioxidants: Adding antioxidants to the formulation (such as vitamin E, ascorbic acid, etc.) can reduce the degradation of HA caused by oxygen.
- Cross-Linked Hyaluronic Acid: Cross-linking HA molecules can significantly increase their stability in solution. Cross-linked HA decomposes more slowly, making it more suitable for long-lasting moisturizing or injectable products.
- Appropriate Preservative Additions: Using the right preservatives can prevent microbial contamination, thereby increasing the stability and shelf life of the product.
- Optimize Solution Concentration: Properly adjusting the concentration of the hyaluronic acid solution can prevent aggregation or precipitation, ensuring a stable and effective product.
Related reading: How to Maintain the Stability of Hyaluronic Acid Products
Conclusion
The solubility of hyaluronic acid is crucial for its application in various products, as it determines the final physical properties and functions of the substance. By employing these techniques, the overall stability of HA in the solution can be significantly enhanced, leading to more effective and long-lasting products in both cosmetics and pharmaceuticals.
Frequently Asked Questions About Hyaluronic Acid Solubility
Here are some common questions about using hyaluronic acid, based on what other users have asked. Hope this makes your work easier!
Q1: Why does my hyaluronic acid powder clump up into "fish eyes" as soon as it hits water? This happens a lot. When the powder touches water, the outside instantly soaks up water and turns into a gel. That gel layer then traps the dry powder inside, making those clumps.
What to do:
- Stir the water really fast so it swirls, then sprinkle the powder in bit by bit. Make sure the powder doesn't hit the water in clumps.
- Never just dump a whole spoon of powder into still water.
Q2: What's the best way to dissolve hyaluronic acid without breaking down its molecular weight? The long-chain structure (molecular weight) is what makes hyaluronic acid work well. Rough handling or fast mixing can snap those chains.
What to do:
- Once the powder is evenly wet and no lumps remain, slow down. Use a gentle stirrer (like a magnetic one) and let it sit for a few hours—or even overnight—to fully dissolve.
- Stay away from high-speed blenders unless you don't mind the molecules getting shorter.
- Don't use hot water—room temperature or cool is better. Heat can damage it.
Q3: How concentrated can I make my hyaluronic acid solution? Why is it so hard to make a strong mix? The thicker it gets, the harder it is to dissolve. Even a 1% solution is pretty gooey. Trying to make it past 2% is tough with normal mixing.
Q4: What kind of water should I use? Stick to deionized or purified water. Tap water or mineral water has stuff like calcium and magnesium in it, which can mess with the hyaluronic acid. That can make your solution cloudy or less thick.
Q5: Why is my hyaluronic acid solution not clear, or why isn't it as thick as I expected? A few things could be going on:
- It's not fully dissolved: You might still have tiny gel bits floating in there. Keep stirring until it's totally clear.
- The molecules got broken: Maybe you mixed too hard, used heat, or the pH was off. That shortens the chains and thins it out.
- The powder isn't pure enough: Low-quality hyaluronic acid or impurities can affect clarity and thickness.
Q6: How should I store my hyaluronic acid solution?
- Keep it in the fridge (around 4°C).
- Always add a preservative—hyaluronic acid can grow bacteria and mold. Something like 0.1–0.3% phenoxyethanol works well.
- Don't freeze and thaw it over and over—that can break down the structure and make it less effective.