Solubility, Viscosity, and Stability of Sodium Hyaluronate
Sodium hyaluronate (sodium salt of hyaluronic acid, HA) is a natural polysaccharide widely found in human tissues such as skin, joints, and the vitreous body of the eye. It possesses various properties, including moisturizing, lubricating, and biocompatibility, making it widely applicable in multiple fields. In practical applications, its solubility, viscosity, and stability are key factors affecting its performance.
Solubility of Sodium Hyaluronate
The solubility of sodium hyaluronate is the foundation of its application. Solubility not only affects its dispersibility in different solutions but also directly influences its bioavailability and efficacy. The solubility is influenced by various factors, including solvent type, temperature, pH, and the molecular weight of hyaluronic acid.
–How Long Does It Take for Sodium Hyaluronate to Dissolve
The solubility of sodium hyaluronate varies in different solvents. In water, it can dissolve quickly, forming a transparent and viscous solution. In glycerol, its solubility is moderate, though not as high as in water. This is why glycerol and hyaluronic acid are often found together in cosmetics. In ethanol, the solubility of hyaluronic acid is low, and it usually requires mixing with other solvents. In acetone, sodium hyaluronate is almost insoluble and is generally not used in such solvents.
Table 1. A comparison of the solubility of sodium hyaluronate in different solvents:
| Water | Oil | Organic Solvent | Ethanol | Methanol | Alcohol | Glycerine | |
| Solubility | High solubility | Insoluble | Very low to insoluble | Very low to insoluble | Very low to insoluble | Very low to insoluble | Moderate solubility |
| Optimal Temperature | 20-40°C | / | / | / | / | / | 30-40°C |
| Optimal pH | 5.5-7.5 | / | / | / | / | / | 5.5-7.5 |
Stanford Chemical Company (SCC) is a trusted supplier of sodium hyaluronate powder. We provide customers with high-purity, non-animal-derived, non-GMO hyaluronic acid powder (including food grade, cosmetic grade, injection grade, medical grade, eye drop grade, cross-linked gel). Enjoy bulk purchase discounts and contract pricing.
–How to Dissolve Sodium Hyaluronate Quickly
In addition to solvent type, several factors influence the solubility of sodium hyaluronate. A previous article discussed this topic in detail. If interested, you can click to read: Solubility of Hyaluronic Acid in Different Solvents and Its Influencing Factors
To accelerate dissolution, the following methods are commonly used:
- Stirring: Mechanical stirring can speed up the dispersion and dissolution of HA in water. The stirring speed and duration significantly affect the dissolution efficiency.
- Heating: Moderate heating (usually not exceeding 60°C) can increase the dissolution rate, but care must be taken to avoid degradation caused by high temperatures. Temperature should be controlled, and prolonged high-temperature treatment should be avoided.
- Premixing: Premixing sodium hyaluronate with a small amount of glycerol or ethanol before diluting with water can improve dissolution efficiency. This method is particularly suitable for preparing high-concentration sodium hyaluronate solutions.
Viscosity of Sodium Hyaluronate
Viscosity is one of the important physical properties of sodium hyaluronate, directly affecting its application in cosmetics and medicine.
–How Viscosity Affects the Efficacy of Sodium Hyaluronate
The viscosity of hyaluronic acid is closely related to its molecular weight. High-viscosity hyaluronic acid forms a protective film on the skin surface, effectively locking in moisture, while low-viscosity sodium hyaluronate penetrates deeper into the skin, providing deep hydration. Viscosity also affects the flowability and distribution uniformity of sodium hyaluronate during injection or application.
–What Factors Affect the Viscosity of HA
Molecular weight, concentration, temperature, pH value.
Higher molecular weight results in higher viscosity. High molecular weight hyaluronic acid has longer molecular chains and stronger intermolecular interactions, leading to higher viscosity.
Higher concentration leads to higher viscosity. In high-concentration sodium hyaluronate solutions, molecules are closer together, resulting in stronger interactions and increased viscosity.
Higher temperatures reduce viscosity. Increased molecular motion at high temperatures weakens intermolecular interactions, causing a decrease in viscosity.
Hyaluronic acid exhibits the most stable viscosity within a pH range of 6-8. Under acidic or alkaline conditions, sodium hyaluronate molecules may undergo hydrolysis or cross-linking, leading to changes in viscosity.
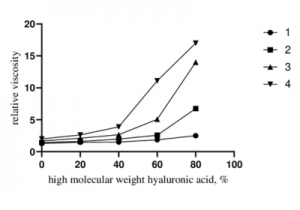
Fig 2. Relationship between the relative viscosity of LMW-HA/HMW-HA aqueous solutions and the HMW-HA content.[1]
Stability of Sodium Hyaluronate
The stability of sodium hyaluronate is another critical factor in its application. Stability not only affects its storage and shelf life but also influences the durability of its efficacy.
The Impact of Temperature on Stability
Hyaluronic acid is prone to degradation at high temperatures, so high-temperature environments should be avoided during storage and use. Typically, sodium hyaluronate is most stable within a temperature range of 4°C to 25°C.
The Impact of pH on Stability
Hyaluronic acid is susceptible to hydrolysis in acidic or alkaline environments, leading to molecular chain breakage. Therefore, maintaining the pH of sodium hyaluronate solutions within the range of 6-8 is crucial for ensuring stability.
The Impact of Light and Oxidation on Stability
Hyaluronic acid is prone to degradation under light and oxidative conditions. Therefore, it should be stored away from light, and antioxidants should be added to extend its stability.
Read more: How to Maintain the Stability of Hyaluronic Acid Products
Table 2 below is a comparison of the stability of HA under different conditions:
| Condition | Stability | Explanation |
| Temperature (4-25°C) | High | Suitable for long-term storage |
| Temperature (>60°C) | Low | Prone to degradation |
| pH 6-8 | High | Suitable for most applications |
| pH <4 or >10 | Low | Prone to hydrolysis |
| Light-protected | High | Prevents degradation |
| Light-exposed | Low | Prone to photodegradation |
| With antioxidants | High | Prevents degradation |
| Without antioxidants | Low | Prone to oxidative degradation |
[1] Saitarly, Svetlana & Pushkarev, Yuriy & Nesterkina, Mariia & Ozturk, Serhat & Salih, Bekir & Kravchenko, Iryna. (2021). Rheological Properties of Hyaluronic Acid Diluted Solutions as Components of Cosmetics. Biointerface Research in Applied Chemistry. 12. 1907-1915. 10.33263/BRIAC122.19071915.