- Home
- Industrial & Lab Chemicals
- Custom Synthesis
- 2-Pyrimidinemethanol
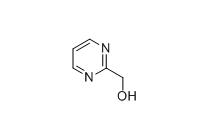
2-Pyrimidinemethanol
CAS Registry Number: 42839-09-8
MF: C5H6N2O
MW: 110.11
Purity: 98%HPLC
- Description
Description
Description
| CAS Registry Number | 42839-09-8 |
|---|---|
| Molecular Formula | C5H6N2O |
| Molecular Weight | 110.11 |
| Purity | 98%HPLC |
2-Pyrimidinemethanol is an organic compound belonging to the pyrimidine derivatives. Its molecular formula is C₆H₇N₃O, and its structure consists of a pyrimidine ring connected to a methanol group. This compound is commonly used as an intermediate in the synthesis of various pharmaceuticals, pesticides, and other organic compounds.
Synonyms: 2-Pyrimidinylmethanol, 2-Aminopyrimidine Methanol, 2-Pyrimidinemethanol
Chemical Properties
| Property | Description |
| Molecular Structure | Pyrimidine ring + hydroxymethyl group (combines aromatic polarity and hydroxyl reactivity) |
| Solubility | Soluble in water, alcohols (e.g., methanol/ethanol), and polar organic solvents (DMF, DMSO) |
| Acid-Base Nature | Weakly basic (pyrimidine nitrogen can be protonated); hydroxyl group shows weak acidity |
| Stability | Relatively stable to light/heat, but the hydroxyl group may oxidize (store sealed in the dark) |
Key Features
- Bifunctional Reactivity:
- Pyrimidine ring acts as a hydrogen bond acceptor (target binding site in drug design)
- Hydroxymethyl group can undergo derivatization (esterification, etherification, oxidation to aldehyde/carboxylic acid, etc.)
- Biocompatibility: Commonly used in pharmaceutical intermediates with low cytotoxicity
- Coordination Ability: Forms complexes with metal ions (e.g., Cu²⁺, Pd²⁺) for catalytic systems
Primary Applications
| Field | Examples |
| Pharmaceutical Synthesis | – Intermediate for antiviral/anticancer drugs (e.g., HIV protease inhibitors) – Antibiotic structural modification (enhancing solubility or activity) |
| Materials Chemistry | – Ligand design (MOFs, homogeneous catalysts) – UV-absorbing materials (pyrimidine conjugated system) |
| Agrochemicals | Synthesis of high-efficiency, low-toxicity pesticides (e.g., pyrimidine-based fungicides) |
| Research Reagents | Used as a building block for heterocyclic compound libraries |